Link: OHDSI – Observational Health Data Sciences and Informatics7
Brief description of the OHDSI initiative and data
The Observational Health Data Sciences and Informatics (OHDSI, pronounced "Odyssey") collaboration is an open-science, multi-stakeholder, interdisciplinary community. Its goal is to use large-scale open-source analytics to generate evidence that promotes better health decisions and care. OHDSI incorporates an international network of researchers and observational health databases with a central coordinating center located at Columbia University. As of November 2021, the community included 2367 researchers from 74 countries, and health records for about 800 million people from around the world.
OHDSI’s health data network is based on the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM), enabling federated analytics amongst collaborators. OHDSI Researchers develop standards8 and open-source software9 to standardize and facilitate analyses. Research within OHDSI includes characterization, medical product safety surveillance, comparative effectiveness research, personalized risk prediction, and methodological research.
The OHDSI model allows researchers and data partners to contribute data without sharing patient-level information and ensures that common analytical code can be applied across databases. Some data partners collaborate on code and then run that code on the data to which they have access.7 Other partners collaborate by running the code without having contributed to the writing of code. OHDSI’s structure and analysis flow diagram is depicted below (Figure 5.1).
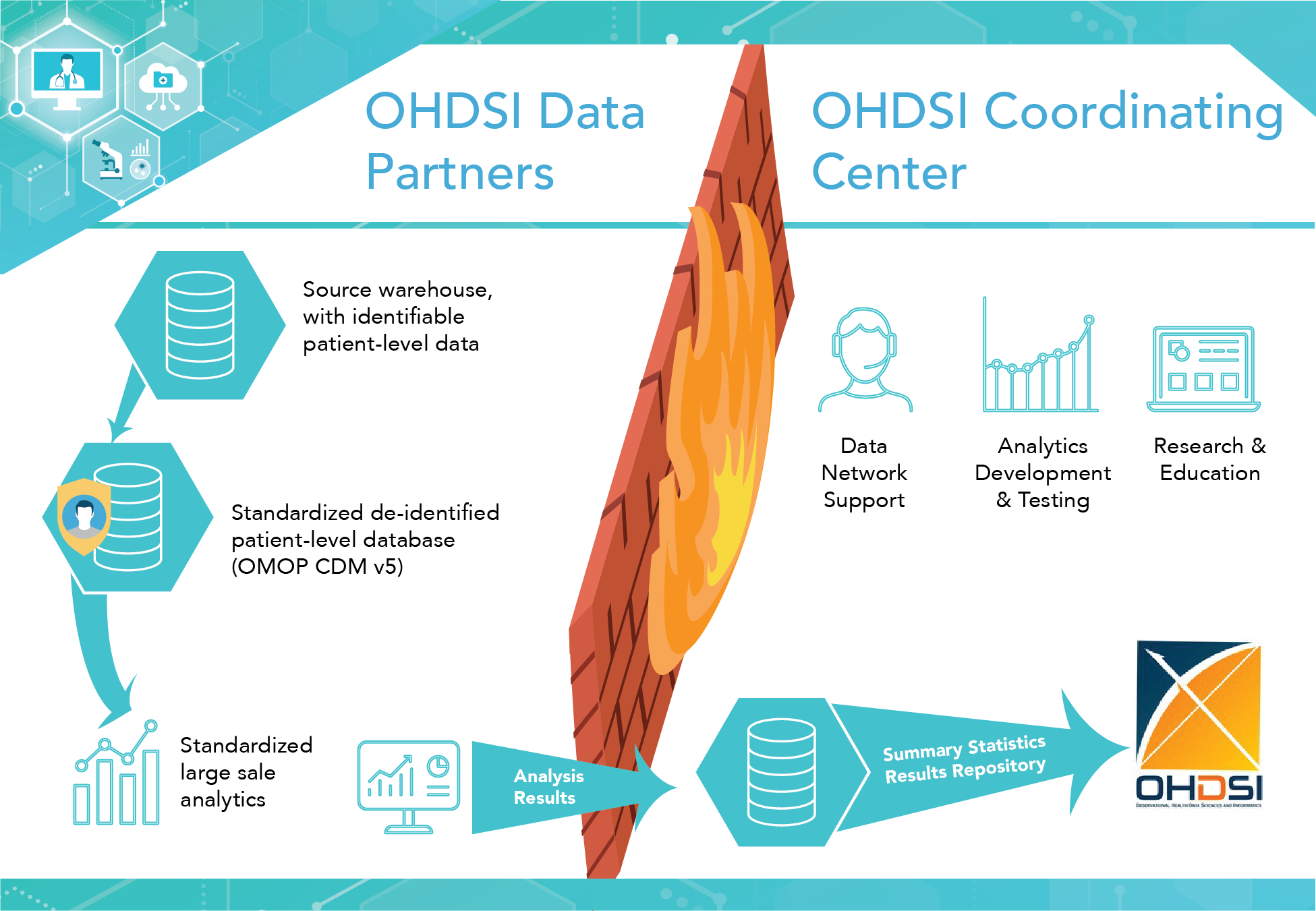
Figure 5.1. OHDSI Structure and Analysis Flow Diagram10
The OHDSI community is closely tied to the European Health Data & Evidence Network (EHDEN) consortium within the Innovative Medicines Initiative (IMI) in Europe. EHDEN11 is developing the required infrastructure for observational health research using the OMOP CDM federated network model across Europe, through a community of data partners and small to medium enterprises (SMEs).
OHDSI and EHDEN COVID-19 observational research: International federated data networks and CDM
Early in 2020, OHDSI and EHDEN joined forces to address urgent research questions related to COVID-19 by building on the existing communities, available data sources, software, and infrastructures created by both initiatives.
From March 26-29, 2021, OHDSI hosted a COVID-19 virtual 4-day study-a-thon12 to inform health care decision-making in response to the global pandemic. Supported by EHDEN, the study-a-thon convened more than 300 researchers from 30 countries, with access to 37 health care databases with COVID-19 patient data.
Fifteen workstream groups were created, each addressing different research questions. Five major milestones were established for each research question: 1) Review relevant literature and develop the study protocol, 2) Develop and evaluate phenotypes, 3) Develop the study packages (these are freely available via the OHDSI GitHub),13 4) Execute these study packages across the available data network, 5) Review findings by clinical experts and disseminate the results.
Phenotypes and cohorts were also defined, with a total of 355 cohorts created. Of these, 114 were reviewed and validated for use in the study-a-thon studies (data.ohdsi.org).13 Execution of studies began almost immediately.
Since March 2020, OHDSI has been focusing on COVID-19 major research in areas which can be systematically examined across the OHDSI network. These include:
- COVID-19 characterization and COVID-19 disease natural history by defining diseases and populations of interest, illustrated by the CHARYBDIS Project (Characterizing Health Associated Risks, and Your Baseline Disease In SARS-COV-2)
- Population level estimation to examine the comparative safety and effectiveness of COVID-19 therapies, addressed by Project SCYLLA (SARS-Cov-2 Large-scale Longitudinal Analyses) which focuses on COVID-19 treatments 1) administered during hospitalization and prior to intensive services, 2) administered during hospitalization after initiating intensive services, and 3) administered after COVID-19 positive testing and prior to hospitalization
- Patient-level prediction, illustrated by study Seek COVER: Development and validation of a personalized risk calculator for COVID-19 outcomes in an international network
Key features characterizing the OHDSI community and related initiatives, such as EHDEN, in their ability to contribute to COVID-19 observational research include:
- Research and data partners multi-stakeholder community with a global geographic reach
- Federated data networks, including EHR, hospital billing data, and insurance claims
- Data harmonization, data using the OMOP CDM
- Established and validated COVID-19 cohorts, with regular data refresh
- Systematic approach to generate evidence on COVID-19
- Open community, open-source (OHDSI Studies · GitHub)13
- Protocols, analysis codes, results publicly available (COVID-19 Updates Page – OHDSI,14OHDSI Studies · GitHub)13
- Pre-prints and scientific publications available
- Possibility to contribute to OHDSI working groups15
- Possibility to propose research questions and lead studies
- Use of study results to inform health care system and regulatory decision making
OHDSI case studies
The OHDSI data network enabled a rapid baseline analysis of COVID-19 in early hotspots using routinely collected primary care EHR data, hospital billing data, and insurance claims, initially from the US, South Korea, and Spain.
One first study described the characteristics of adults hospitalized with COVID-19 by training a predictive model for influenza and testing it against COVID-19. An analysis of data from 34,128 COVID-19 patients compared to 84,585 individuals hospitalized with influenza in 2014–2019 found that hospitalized COVID-19 patients were more likely to be male, younger, and have fewer comorbidities and less medication use. Their conclusion was that “while protecting groups vulnerable to influenza is likely a useful starting point in the response to COVID-19, strategies will likely need to be broadened to reflect the particular characteristics of individuals being hospitalized with COVID-19.”16
When angiotensin-converting enzyme inhibitors and angiotensin receptor blockers had been postulated to increase susceptibility to COVID-19, an OHDSI-based study found no clinically significant increased risk of COVID-19 diagnosis or hospital admission-related outcomes, “suggesting users should not discontinue or change their treatment to decrease their risk of COVID-19.”17
In response to the emergency use authorization of hydroxychloroquine for COVID-19, OHDSI was used to conduct a large cohort study of rheumatoid arthritis patients (without COVID-19) who were taking hydroxychloroquine. The study found that the addition of azithromycin to hydroxychloroquine appeared to be associated with increased risk of heart failure and cardiovascular mortality.18
Other studies have examined COVID-19 among children and adolescents,19 patients with prevalent autoimmune diseases,20 patients with obesity,21 and patients with a history of cancer.22The use of repurposed and adjuvant drugs in patients hospitalized with COVID-1923 was also examined, and the OHDSI framework was used to externally assess the validity of a COVID-19 risk model.24 An updated list of preprints and published OHDSI studies can be found at the OHDSI webpage.14
OHDSI impact and perspective
OHDSI has contributed to the understanding of key disease knowledge gaps initially posed by the pandemic. Results from studies conducted by OHDSI have informed clinicians, health care systems, and regulators. These results have contributed to better management of COVID-19 patients during the acute phases of the pandemic and have permitted real-time evaluation of COVID-19 therapies by regulators.
Daniel Prieto-Alhambra, a key contributor to OHDSI, describes it as “a very inclusive community in which basically anyone is invited to come and join and work with us. You just join the forums, attend one of the calls, and join a working group. It’s an open science community.” EHDEN, which he finds is often conflated with OHDSI in discussions, has a different but complementary aim “to generate a new transparent, fair, data access network in Europe. They work very nicely together.” Prieto-Alhambra describes a parallel process to OHDSI’s study-a-thon” that he calls an “Evidence-a-Thon.” New data partners in the EHDEN network, are asked to run one study that has already been completed in other databases, review the results, and write up the findings. The process not only tests the CDM, but also engages new data partners immediately.
OHDSI community researchers have defined a “long research agenda” to address future COVID-19 research needs. That agenda includes the characterization of COVID-19 over time (as a condition that changes with vaccinations and variants), long COVID-19 or persistent COVID-19, the sequelae of COVID-19 (organ failure, cardiovascular disease, and thrombotic events), the impact of current treatments, and new treatments that will arise to treat the virus and its complications. It also includes questions related to vaccine safety and effectiveness, plus community-level factors and non-pharmacological interventions.
